July 18, 2023
OVERCOMING OBSTACLES IN DOWNSTREAM BIOPROCESSING OF AAV-BASED GENE THERAPY PRODUCTS
Author: Quentin BAZOT
Adeno-associated virus (AAV) is a small virus used in gene therapy applications. The demand for AAV clinical trials and approved therapeutic applications is increasing due to this vector’s overall success and potential. With the shift beyond ultra rare indications and the use of new engineered synthetic AAV capsids, there is a need for new, productive, scalable and easy-to-implement AAV platform processes compatible with different AAV serotypes. However, the establishment of such productions and purifications platforms comes with obstacles that the manufacturers need to overcome. This article will focus on the challenges faced during the purification step of AAV manufacturing also called the downstream processing (DSP).
AAV is a DNA virus surrounded by proteins that we call capsid proteins. Different AAV serotypes have been identified, differing in their capsids component and thus displaying different tissue tropism. AAVs are used as vectors, meaning they can carry therapeutic genes into human cells, where the therapeutic genes can then be expressed and used to treat genetic diseases. Over the past decade gene therapy has been rapidly growing, with numerous companies developing AAV-based treatments for a variety of genetic disorders(1). Some of the most promising AAV gene therapies include treatments for inherited retinal diseases, spinal muscular atrophy, Parkinson’s disease and hemophilia. To help the industry and tackle the different AAV manufacturing challenges, ABL, a CDMO specialized in the development and GMP manufacturing of viral vectors, is working on the establishment of an AAV Platform. AAV manufacturing processes can be split into two major steps, the upstream and the downstream processing (Figure 1).
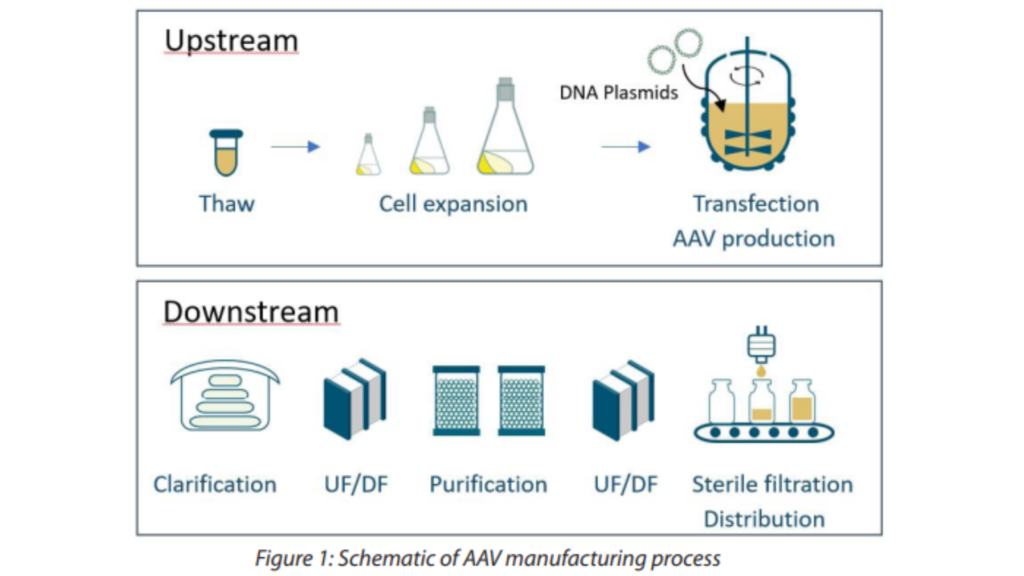
1. The upstream processing (USP)
The USP is where AAV is going to be produced. It is the cell culture stage from the cell thaw to the production in bioreactor. For AAV production there are different production systems available based on different cell types (adherent, suspension) from transient AAV production using DNA plasmid transfection to the stable production using stable cell lines. The transient production system using DNA transfection is the most used system (as seen in Figure 1) as it offers flexibility and a large room for optimization. Each of these approaches for AAV production will use different reagents leading to different challenges for the purification of the vectors at the DSP step.
2. The downstream processing (DSP)
The DSP is the purification process that aims at separating the viral vector of interest from the impurities produced during the manufacturing process. They are two types of impurities, the process and product related impurities:
- The process related impurities arise from the manufacturing process (USP and DSP). The main impurities are composed for the upstream processing of the cell debris, cell culture media component, the transfection reagent, the DNA plasmids as well as host cell DNA and proteins. In addition to upstream process related impurities the downstream process related impurities that are going to be introduced while trying to remove the upstream process related impurities will also need to be cleaned. Those DSP impurities are related to the different buffers and chromatography media used during the purification steps.
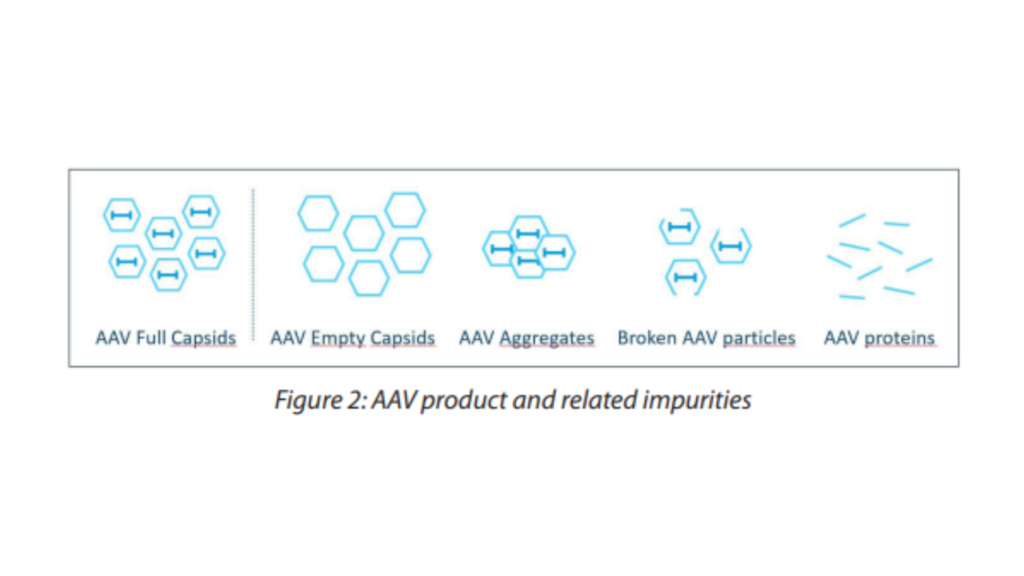
The product related impurities are directly correlated to the product of interest, here AAV (See Figure 2). Indeed, the particle of interest at the end of the manufacturing process will be an active AAV particle, not damaged and containing the therapeutic gene (AAV Full capsids). However, most of the AAV produced during the manufacturing process will be composed of damaged particles, AAV proteins, AAV aggregates and what is called “empty capsids’ where no genome has been encapsidated.
The downstream processing will also depend on the AAV serotypes with all AAV serotypes behaving differently during their production and purification. One good example is their distribution after production, some AAV serotypes being mostly secreted (extracellular) while other serotypes being mostly intracellular requiring a lyse step to get all the AAV produced. As discussed earlier the downstream processes will be different depending on the AAV production system used and the AAV serotypes. However, all DSP have a similar workflow composed of three main steps (see figure 1).
- A Harvest and Clarification Step where AAV is going to be released (lysis step when needed) and where the bulk will be clarified through filtration (clarification step). This step aims at removing the process related impurities that are the cell debris and organelles.
- A purification step that can be divided into two main stages which are the capture and the polishing. Purification techniques such as ultracentrifugation and chromatography are used. The capture step aims at removing the process related impurities that are the host cell DNA, RNA, proteins and transfected DNA, while the polishing aims at removing the product related impurities such as the defective AAV forms (damaged & empty) and AAV proteins.
- A concentration / formulation and final filtration step to get the virus into its appropriate state for storage and patient administration.
In addition to those main steps an ultrafiltration (UF) and/or Diafiltration (DF) step to concentrate or exchange the buffer of the product can be used after clarification and/or between the chromatography steps.
3. What are then the manufacturing challenges of the AAV downstream processing?
The manufacturing requirement is for a robust removal of product and process related impurities (complying to the regulatory requirements on safety) while retaining high titer of infectious AAV particles. One other challenge is to have a streamlined process leading to a costeffective and secure supply of GMP material.
The first challenge is the legacy based on centrifugation steps for clarification and purification. Indeed, a few years ago most AAV processes were coming from academic laboratories using centrifugation strategies for AAV purification. While the centrifugation technique works well at small scale, it is not ideal at large scale and in a GMP environment. Ultracentrifugation can be used as a great example. Ultracentrifugation is used a lot as a polishing step to separate the two closely related AAV species that are the empty and full AAV particles. This technique works well at the lab-scale, but the scalability is difficult leading to long processing time and potential manufacturability and safety issues. The industry is now using depth filtration for the clarification step and chromatography techniques for the purification steps. Those are much more scalable and cost effective than centrifugations. Furthermore, chromatography is now a well-recognized technique for AAV purification.
Another challenge is the number of process steps along the downstream processing. Indeed, the more steps there are, the less overall viral vector recovery will be. If we take the number of DSP steps shown in the figure 1, there are a total of 6 steps that are the clarification, UF/DF, Capture, Polishing, UF/DF, and final filtration steps. If each step leads to 70% recovery, the overall recovery for the downstream processing will only be 17%. This means more than 80% of the product is lost during the purification. There is an urge to reduce the number of processing steps in a classic AAV downstream process while increasing the efficiency of each step individually.
Technical downstream processing challenges are seen at each DSP step of an AAV purification process. For the clarification steps, the choice of filter to be used must be carefully selected depending on the AAV feed (from the upstream processing). Indeed, different filters will give different results depending on the nature of the clarification membrane and the pore size selected. The goal of the clarification step is to ensure a high viral genome recovery while reducing host cell debris and contaminants.
3.a. Filtration screening Study
Based on suppliers’ data we chose and screened different filters for the clarification of AAV products. Lysed cell culture bulk (treated with nuclease) was used to screen a total of six clarification trains with the aim of finding the best filters based on AAV recovery (viral genome – vg), filter capacity and turbidity reduction. We decided to test six clarification trains (using the same flow rate) with either one or two filters during what is called a pressure max study where the concept is to filter the product, here AAV lysate, and stop the filtration when the pressure reaches the maximum pressure set up. Our pressure target in this screening was set to 0,8 bars.
The results can be seen in table 1. The AAV recoveries obtained from the six runs were very different from 40 to 90% recovery. Interestingly, the turbidity reduction was high for all filters tested with less than 20 Nephelometric turbidity units (NTU) at the end of the clarification (the starting material was 500 NTU).
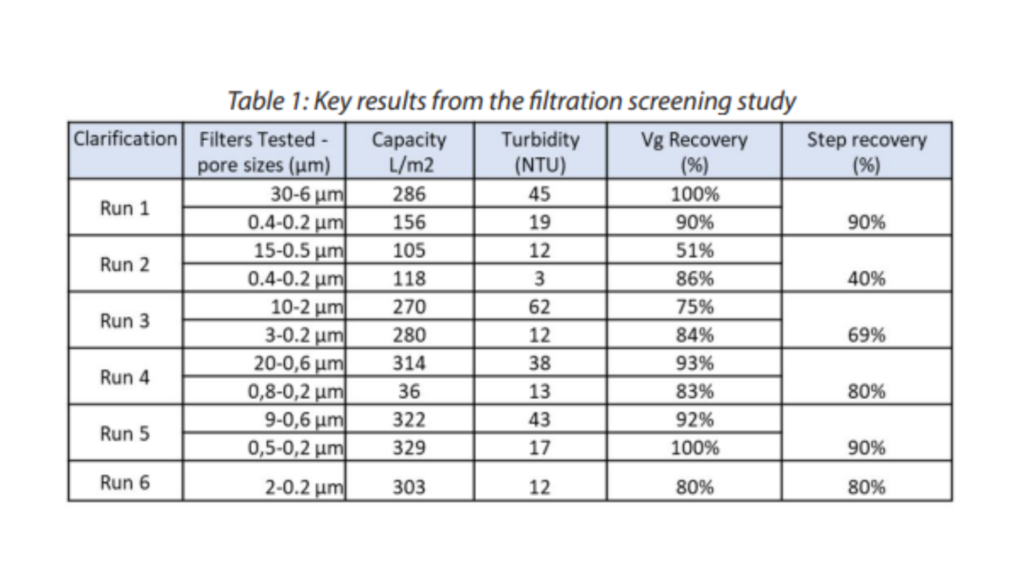
It is striking that the filter capacity ranges from 100 to more than 300 L per square meter of filters. It is important to note that the second filter used for the run 4 was ten times bigger in surface compared to all filters which explains the low filter capacity of 36 L/m2. This filter capacity is a very important parameter when choosing its filter train as it will be crucial for the process scale up.
3.b. Chromatography technique for AAV Capture and Polishing
After the clarification step, the AAV particles need to be purified and selectively removed from the host cell DNA/protein and empty AAV particles. For these purification steps, chromatography has become over the years the method of choice. Different types of chromatography exist and can be used for AAV purification based on either affinity to a ligand, net charge, hydrophobicity, or size. All chromatography can be used for AAV purification however two types of chromatography are principally used for each of the two AAV purification steps, the affinity chromatography for the Capture step and the Anion Exchange (AEX) for the polishing step. Furthermore, most AAV purification processes are serotypes specific, requiring the design of a new process for each serotype used. There is then a clear need to establish an AAV purification process serotypes independent.
Affinity chromatography is used for the AAV Capture step and relies on the interaction of AAV with a ligand. Interestingly, over the years, a lot of suppliers have worked and developed different affinity resins for AAV purification, each of them having their pros and cons. If we focus on the challenges here, which is the development of a purification process working for various serotypes, one resin stands out from the others, the POROS™ CaptureSelect™ AAVX Affinity Resin. This resin is a pan-serotype affinity resin that binds wild type and novel or engineered AAV capsids. Using this resin, we were able to recover more than 95% of AAV for AAV2 and AAV8 serotypes using the same process with the same elution buffer. ABL is currently assessing this resin for a few other serotypes.
The use of affinity resin for AAV purification is very promising but also comes with potential challenges which are the possible leakage of ligands and the high cost of the resin. Affinity resin can be re-used however this re-use should be well documented and validated in order to use this strategy in AAV GMP manufacturing.
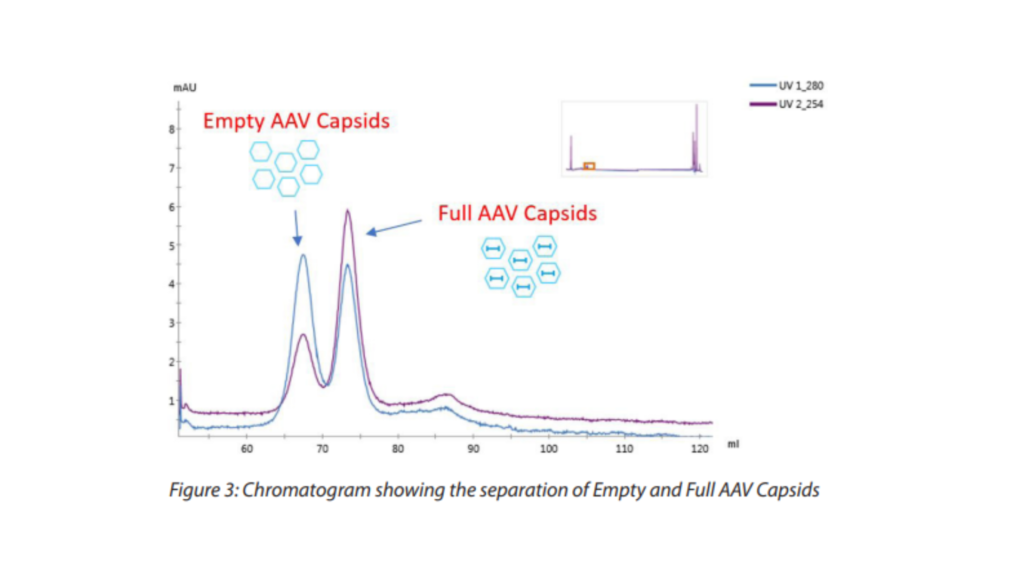
Anion Exchange chromatography is largely used for the polishing step in order to separate the “full” from the “empty” AAV capsids. The polishing is then a crucial step aiming at enriching the final product with the “full” active AAV particles. Indeed, if high levels of empty capsids contaminate the final product this would lead to a decrease of the therapy efficacy. In addition to the empty capsids, the partially filled capsids containing truncated genome or process related impurities packed into the genome should be removed at this step. The AEX chromatography is based on net charge of the particle of interest, net charge that will be different from the Empty and Full AAV particles due to the presence or absence of genomic DNA (leading to a difference in their Isoelectric point). The isoelectric point of empty and full AAV capsids are around 6,3 and 5,9 respectively.
Mixed with a high pH Buffer (usually around pH 9) the AAV capsids net charge is negative, with the full capsids being more charged than the empty capsids. The closely related AAV species can then be eluted using a salt gradient, where the empty capsids will elute first as less negatively charged compared to the full capsids (see figure 3). Initial assessment of this separation can be seen on the chromatogram level when comparing the ultraviolet (UV) wavelength at 280 nm, for Protein, with the UV at 260 nm, for DNA. When the peak in the chromatogram shows a UV 280 nm signal bigger than the UV 260 nm signal this indicates that the peak is predominately empty. On the other end when the UV 260 nm signal is higher than the UV 280 nm the AAV capsids are predominantly full (compare the peaks on the chromatogram – Figure 3).
Interestingly, there exists different chromatography media that can be used for AEX chromatography, the conventional resins and the membrane and monolith media. AEX chromatography is an easily scalable and cost-effective technology. The overall challenge with the polishing step is that it requires optimization for each serotype, gene of interest, production system used.
4. Conclusion
The main challenge in AAV manufacturing is to purify a high volume of AAV with the key requirements that are high purity, high titer and high potency. If we focus on the overall AAV downstream manufacturing, there is an urge to move away from poorly scalable centrifugationbased techniques. We also need a simplification of the process design, reducing the number of DSP steps leading to an improvement of the overall yield. There is also a need to drive down the cost of goods of AAV processes. Interestingly, a lot of new technologies are emerging for viral vector purification(2) which should help us tackle the main challenges we faced in the AAV downstream manufacturing. One clear area of improvement in AAV downstream processing is the chromatography steps where new bead-based affinity chromatography resins have been recently launched for AAV capture step (AVIPure®-AAV Affinity Resins). These new resins are serotype specific but have the great advantages of being easily cleanable with NaOH helping the reuse strategy of the resin. New chromatography media were also developed offering a good alternative to the standard resins. Those media (membrane & monolith) offer a fast mass transfer allowing high flow rate (compared to low flow rate using resins) and a quick process. However, the downside of those new chromatography media is that they offer a reduced surface area compared to resins. It is then a delicate balance to find between surface area and flow rate, but one can imagine that in the near future new technologies will emerge allowing high surface area and high flow rate.
New technologies as alternatives to Capture chromatography are also developed and are promising. This is the case for the IsoTagTMAAV, a new method to capture AAV using a fusion protein. Under the right condition the protein will bind AAV and aggregate to form big complexes that can be easily separated from the impurities in the lysed harvest using a Tangential Flow Filtration (TFF) device. After the AAV capture using the IsoTagTM-AAV, the complexes are re-solubilized and the AAV eluted and recovered. This AAV purification process is quick, cost effective, works for different serotypes and is easily scalable. We are now entering a very exciting time where new technologies are being developed to tackle the AAV downstream processing challenges. ABL is already assessing and optimizing these new technologies to provide new solutions for AAV manufacturing.
