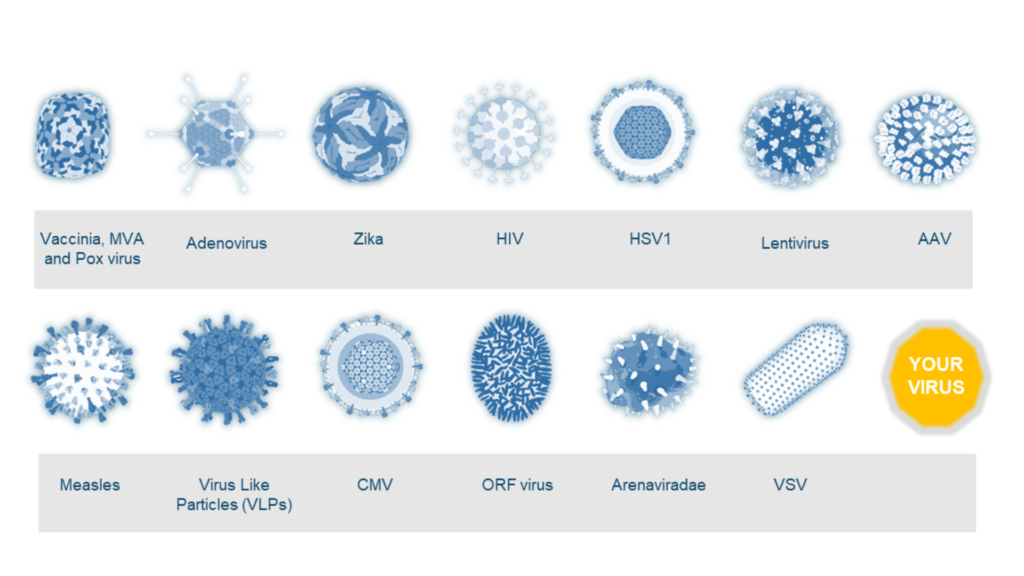
VIRAL VECTORS PORTFOLIO
ABL has an extensive experience in manufacturing of a broad range of viral vector-based therapies and can support development of your program with innovative solutions and state-of-the art technology platforms.
Our approach is platform-agnostic and is based on applying internal know-how acquired across time (being active in this field for decades) and space (by cumulative expertise from 3 different sites).
With a strong technical background and sustained by a regulatory track record in process development, scale-up & GMP manufacturing of viral vectors for use in multitude of therapy indications, our expertise is vast and includes Vaccines, Oncolytic Viruses (OV), Gene Therapy products and other Immunotherapies.

As a pioneer, we have been developing and producing viral vectors since the 1990s. We started with Pox Virus, MVA, Vaccinia and oncolytic viruses to support our clients’ clinical trials with the ultimate goal of fighting smallpox and several types of cancer.
We have continuously added new viruses to our portfolio, including manufacturing of AAVs in 2016 through adherent and suspension processes. Based on this strong expertise in virology and cell culture, in 2022 we decided to develop our high predictable yield and versatile AAV platform to manufacture different serotypes.
Thanks to our expertise of over 25 years, we are the right partner to support your Vaccinia and MVA projects with a successful track record in delivering GMP clinical batches up to Commercial through adherent and suspension cell culture.
GENE THERAPY
At ABL, we support Gene Therapy companies and help them discovering new treatments for diseases with unmet medical needs.
AAV has emerged as a leading vector for gene delivery for treating various diseases due to its safety profile and efficient transduction of various target tissues. With the shift beyond ultrarare indications and the use of engineered synthetic AAV capsids, there is a need for new, scalable and easy-to-implement AAV platform processes compatible with different AAV serotypes.
We know that fast-paced roadmap and affordable development costs are critical to ensure sustainability and high success rate of Gene Therapy clinical development programs.
That’s why ABL decided to develop a flexible and versatile platform including suspension-based AAV upstream process and universal chromatography solutions, providing efficient and cost-effective solution from labscale process development to large scale production.
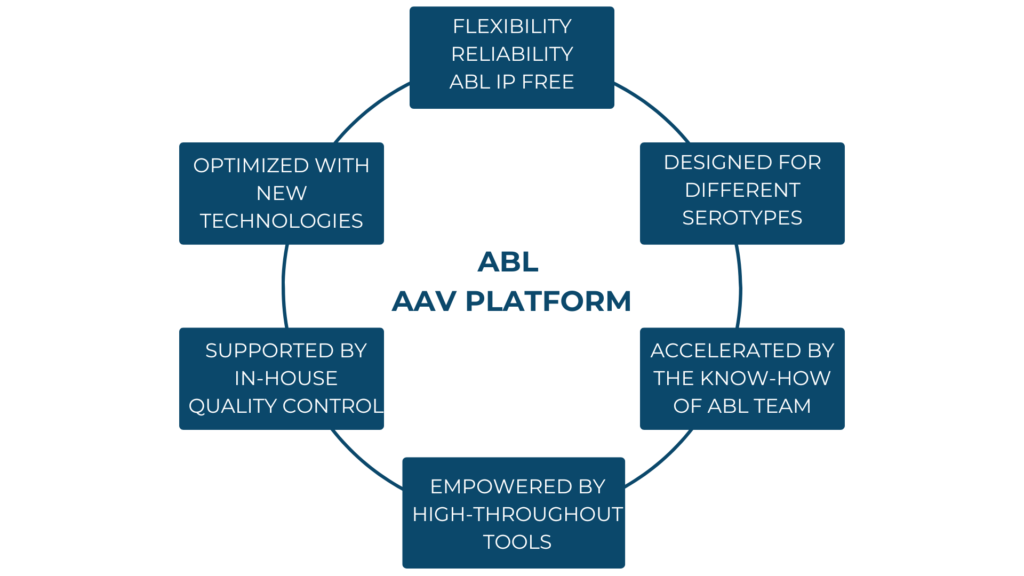
Flexibility, Reliability, ABL IP Free: our platform is open for your plasmid and cell lines. We can also integrate commercial plasmid system and commercial cell lines (incl GMP grade) through partnerships with well-established vendors.
Optimised with new technologies: want to reach the next step of transfection ? We can use Synthetic DNA to bring you AAV process to high performance levels.
Supported by in-house quality control: we are proud to support our projects with in-house QC capabilities that allows us to perform more than 85% of the testing internally. Learn more about our QC capabilities.
Empowered by high-throughout tools: because time is crucial for you and your patients, we have implemented highly-performant development tools like Ambr® 250 from Sartorius, Akta Avant 150 from Cytiva, SamuxMP from Refeyn, Naica dPCR system from STILLA, ELLA from BioTechne. Those technologies help saving months in setting the best conditions for AAV manufacturing.
Accelerated by the know-how of ABL team: you will have access to our team of experts (scientists, engineers, technicians) that gained experience through evaluation of Double vs Triple plasmid systems, assessment of several transfection reagents, comparison of different culture media, optimization of lysis buffers…
Designed for different serotypes: whatever your target and the AAV serotype you chose, our platform has been designed on a large panel of serotypes.
To learn more about the challenges of building a platform in 2023, please read our White Paper.